在加拿大销售的任何食品或饮料必须符合加拿大卫生部的安全和营养质量标准。加拿大对食品标签也有严格的规定。
加拿大服务
加拿大食品检验局执行这些法规。如果不符合安全和质量标准,或食品和饮料产品标签不正确,则意味着这些产品不能在加拿大市场销售,并可能导致法律诉讼。
《加拿大安全食品法》(SFCA)旨在将食品和饮料的不同部分的法规合并为一个整体法规。这些新法规中的一些重要变化是增加了食品原料的可追溯性,并确保进口产品符合国内食品安全要求。Cosmereg可以支持您的业务,为这些更改做好准备。
加拿大卫生部要求食品生产商密切关注其供应链,以降低生产过程中使用欺诈成分的风险。Cosmereg可以帮助您的企业制定一个保护您的产品免受食品欺诈的计划。
全球食品安全倡议(GFSI)是一项旨在提高全球食品安全和质量的多国合作。食品安全认证计划(CPO)必须达到高标准才能获得GFSI认证。Cosmereg可以帮助您的企业选择一个全球食品安全认证管理系统。
要将食品和饮料产品作为清真食品、有机食品、无转基因食品或无麸质食品销售,必须使用经认证的成分以特定方式生产和加工每种产品。正确标记这些产品也非常重要。Cosmereg可以帮助您申请认证,并确保您的食品标签符合加拿大卫生条例。
为了在加拿大销售某些产品,食品和饮料生产商必须向加拿大食品检验局(CFIA)注册。联邦注册机构必须符合食品安全的高标准。Cosmereg可以帮助您的企业满足注册要求。
2016年,加拿大食品标签法规发生了变化。现在食品和饮料产品必须包括有关标准的摄入量信息,以及每种摄入的营养信息。Cosmereg可以帮助您的公司确保所有食品和饮料产品符合最新法规。
临时市场授权(TMA)是尚未获得市场准入批准的产品的一种方式。许多NHP最初是在加拿大的TMA下销售的。Cosmereg可以支持您申请TMA,帮助您的产品尽快上市。
在加拿大销售新产品或含有新成分的产品之前,必须向加拿大卫生部提交一份新的食品通知。Cosmereg可以帮助您完成新的食品通知,确保您的业务符合加拿大法规。
在加拿大,所有婴儿配方奶粉均采用特殊的GMP。在加拿大销售任何婴儿食品或配方奶粉之前,必须经过加拿大卫生部的详细安全评估和营养质量评估。Cosmereg可以协助这一过程,以确保您的产品符合加拿大卫生部的高标准。
在加拿大,食品包装也受到密切监控。包装中使用的任何材料必须自愿提交给加拿大卫生部进行市场前评估。Cosmereg可以在这个过程中支持您的业务。
审计是维护食品安全的重要组成部分。加拿大卫生部规定,食品和饮料供应商必须在其药品生产质量管理规范中进行定期审计。Cosmereg提供完整的食品安全和质量体系审计服务。
根据政府规定,在加拿大销售的产品上的食品标签必须是准确的,并且有英语和法语两种语言。Cosmereg可以通过我们的食品标签咨询服务帮助您完成这一过程。

Regulatory strategic planning and product classification
The first step towards authorization of Medical Devices in Canada is classification based on their level of risk. Class I devices do not require a license. However, producers of these devices require an Establishment License. Cosmereg can help your business to determine which category your medical devices fit into, and support you in applying for the appropriate license.

Organize and manage meeting with the Therapeutic Products Directorate (TPD)
Medical Devices are regulated by the Therapeutic Products Directorate (TPD). Cosmereg can assist your company by organizing and managing meetings with the TPD, for pre-market approval of Medical Devices.

Prepare and file CTA’s (Clinical Trial Applications)
In order to market a Medical Device in Canada for use in a Medical Trial, a Clinical Trial Application (CTA) must first be submitted, just as in pharmaceutical trials. Cosmereg can assist your company with this application process.
Prepare and file Class II, III and IV applications
Class II, III and IV medical devices require a license to be sold in Canada. In order to obtain a license, a Medical Device License Application must be submitted to the TPD. The amount of information required for this application varies according to the device. Cosmereg provides a full Medical Device License Application service.
Provide application support during TPD review
Sometimes, applications are not immediately approved by the TPD – this may be due to a lack of information included in the application documents. Manufacturers of Medical Devices have the right to appeal decisions and re-submit their applications. Cosmereg can assist your business in ensuring that all the required information is included in your application, before it is reviewed by the TPD.
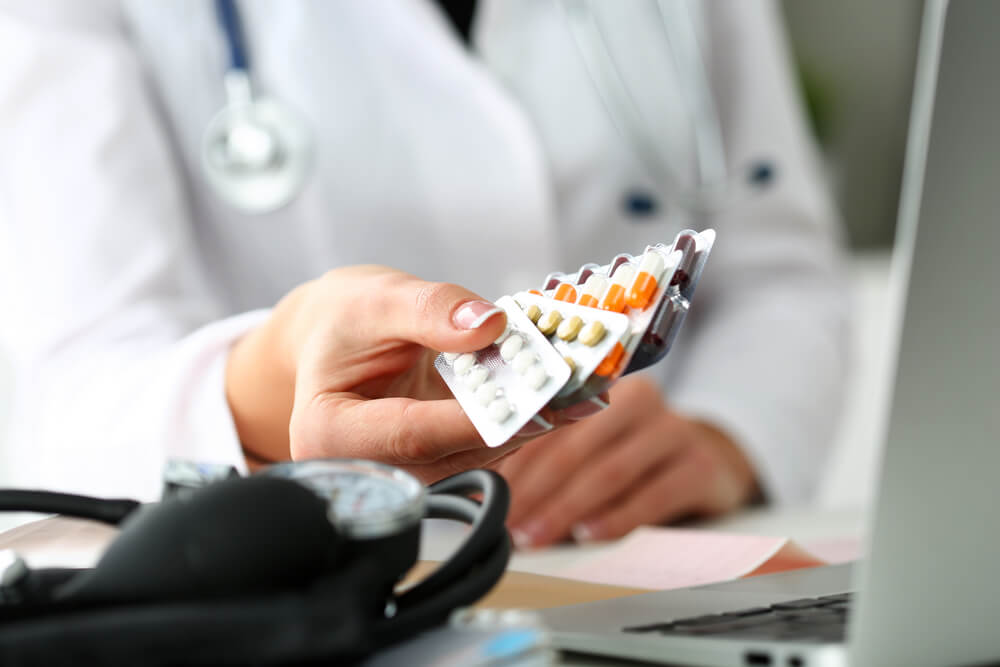
Perform Quality System audits
Manufacturers of Medical Devices must submit a Quality System Certificate to Health Canada. This is to prove that the device complies with industry regulations for its manufacture. Audits of product manufacture and quality are an essential component in ensuring that Medical Devices meet Health Canada standards. Cosmereg can assist your business in designing and conducting these audits.

Provide label and advertising review
Just as with food products or cosmetics, Health Canada stipulates that Medical Devices must be appropriately labelled. Medical Devices must not be sold or advertised in a manner that is misleading to the public. Cosmereg can assist your company to ensure that labels and promotional materials comply with Health Canada regulations.

Resolve regulatory compliance issues
Compliance with Canadian Medical Device regulations can sometimes be a complicated process. Cosmereg provides a full service for regulatory compliance and can assist your business in every step of the process.
不知道该选择什么?
今天就联系我们,我们会咨询你
常问问题
根据《生物恐怖主义法》(2002年),涉及制造、加工、包装或储存人类或动物食用食品的设施必须向FDA登记。