US FDA OTC Monograph User Fee for FY 2022
The US Food and Drug Administration (FDA) announced new Over-the-Counter (OTC) Monograph consumer fees for fiscal year (FY) 2022. The OTC Monograph User Fee Program (OMUFA), which was created by the passage of the March 2020 CARES Act, would require covered OTC drug facilities to pay an annual facility fee starting this year. According to the FDA, facility fees for FY 2022 are due by June the 1st 2022.
This replaces a previous FDA Notice from December 2020 that was removed.
Who is responsible for OMUFA Facility Fees?
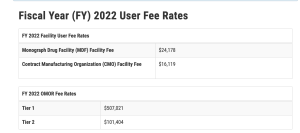
An annual monograph drug facility (MDF) fee will be expected of facilities that produce or process a finished dosage type of an OTC monograph drug. For FY 2022, the MDF fee is $24,178. Contract manufacturing organizations (CMOs), which are MDFs whose owners or affiliates do not market their finished medication directly to wholesalers, manufacturers, or customers, must pay two-thirds of the total cost. CMOs, which are MDFs where the owner or affiliates do not market their finished medication directly to wholesalers, manufacturers, or customers, must pay two-thirds of the normal MDF rate. For fiscal year 2022, the CMO charge is $16,119.
Facilities that either manufacture active pharmaceutical ingredients (APIs), clinical research materials, perform testing, or put outer packaging on already packaged goods for use in a kit are exempt from the OMUFA fees. Moreover, facility fees are also waived if a drug establishment’s registration shows that it has stopped all activities related to OTC monograph drugs by December 31, 2019.
Help with OMUFA & Other OTC Assessment Criteria
If you have doubts on how to classify your establishment or interested in register your OTC product establishment Cosmereg can assist you in each step. For more information about Cares Act just contact us +17273509380 or info@cosmereg.com.