FDA registration requirements to import Face Mask
Guidelines to import face mask and FDA registration
Non-surgical Face mask (no EUA required)
- Register as an importer, and have your manufacturer the FDA registration as a medical device manufacturing establishment with FDA. List the medical device product that you intend to import. (FDA’s fee is $5,236, separate for each company. So each site must re-register and pay the fee annually between October 1 and December 31st)
- Revise your labels to state “Protective Face Mask” or similar
- Add FDA required wording/warnings
- Import when registrations and listings are complete, and when labels have been updated.
Surgical Face mask (EUA)
-
- To apply for the EUA and revise your labels to say “Surgical Mask” or “N95 Mask”. But First, you have to check the product code for your mask to check if you need FDA product clearance and NIOSH approval. All masks claiming to prevent specific diseases, or have antimicrobial and antiviral claims will be considered class II device and requires FDA clearance. For example masks code FXX, OUK or ORW.
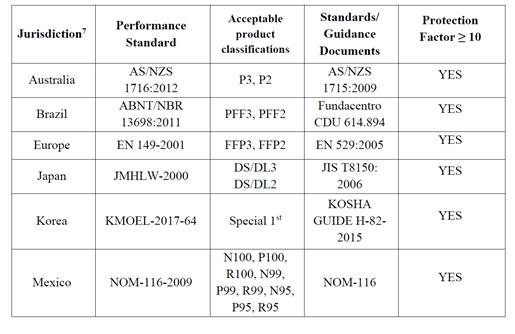
here’s the list of requirements for the EUA. The manufacturer can have proof that they meet the following standards in the table below.
If you don’t have proof of those design and testing standards, you can have proof of approval or certification from:
- European CE Mark
- Australian Register of Therapeutic Goods (ARTG) Certificate of Inclusion
- Health Canada Licence
- Japan Pharmaceuticals and Medical Device (PMDA)/Ministry of Health Labour and Welfare (MHLW)
Chinese manufacturers need the certificate from NMPA, apply for the EUA, and revise labels so to say “Surgical Mask” or “N95 Mask”.
-
- Guidance has been provided by the FDA for the COVID 19 emergency here on this link
https://www.fda.gov/media/136449/download
-
so it is also possible to contact directly by email
CDRH-COVID19-SurgicalMasks@fda.hhs.gov
-
-
- If you intend to sell PPE in the USA and not sure about the procedure and regulations please contact our
-
-
- or call us +1 727 3509380.