REGULATORY NEWS AND RESOURCES
- All
- Canada Cosmetic Regulations
- Canada Dietary Supplement NHP NPN
- Dubai Cosmetic Regulations
- EU Cosmetic Regulations
- International Reuglatory News
- UK Cosmetic Regulations
- Uncategorized
- US FDA Dietary Supplements
- US FDA food regulations
- US FDA Medical Devices
- US FDA OTC regulations
- USA Cosmetic Regulations
All
- All
- Canada Cosmetic Regulations
- Canada Dietary Supplement NHP NPN
- Dubai Cosmetic Regulations
- EU Cosmetic Regulations
- International Reuglatory News
- UK Cosmetic Regulations
- Uncategorized
- US FDA Dietary Supplements
- US FDA food regulations
- US FDA Medical Devices
- US FDA OTC regulations
- USA Cosmetic Regulations
14 US Agent Questions to Know to Meet Medical Device Regulations
Cosmereg
February 20, 2023
14 Key Questions About US Agents to Ensure Compliance with Medical Device Regulations Medical device regulations must be met if you want to sell your ...
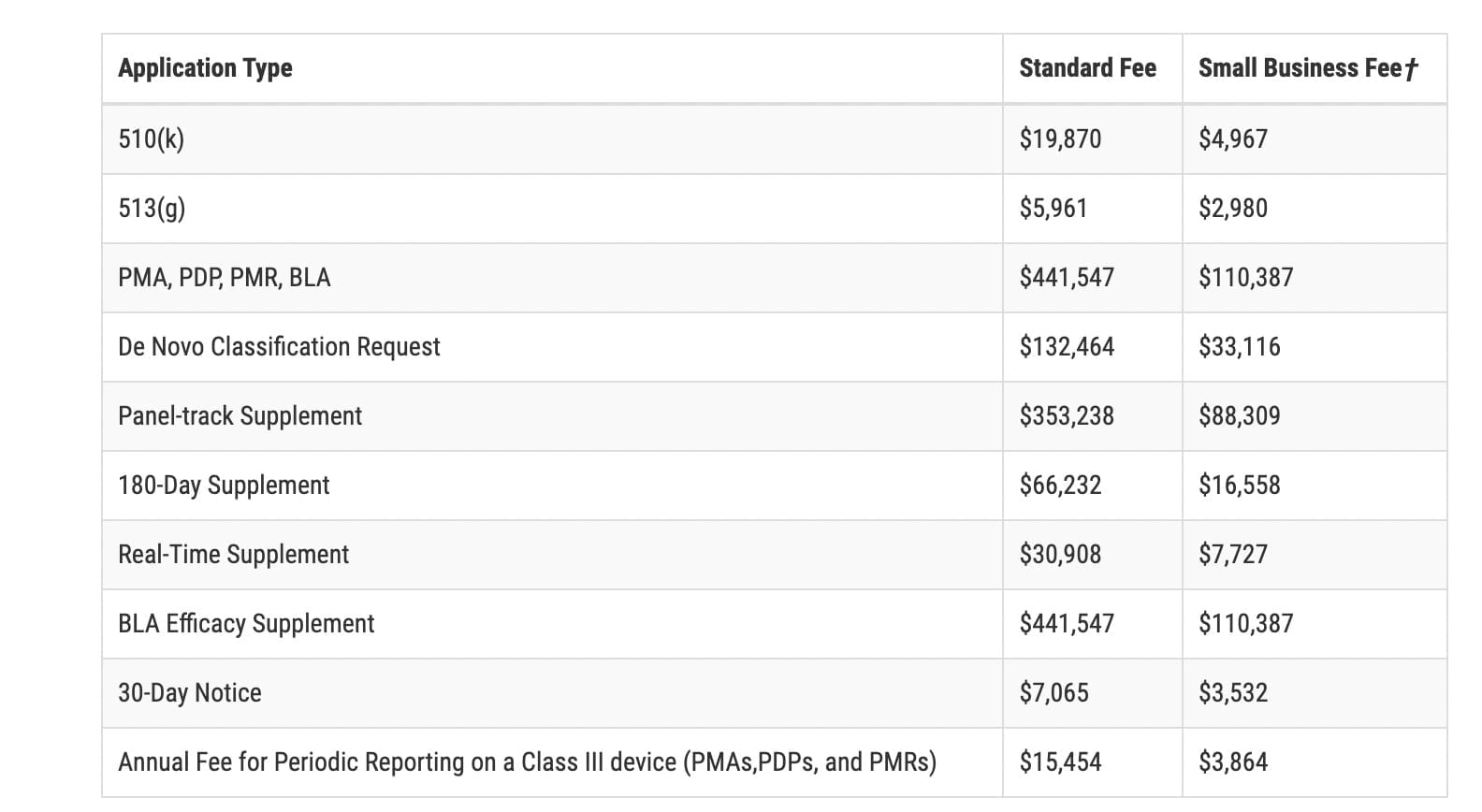
Latest Medical Device User Fee Amendment (2023)
Cosmereg
February 5, 2023
FDA: 2023 Medical Device User Fees Announcement Medical device user fees for the Fiscal Year 2023 are now available following an announcement from the U.S. ...
Key Developments in EU and UK Regulations: Early 2023 Updates
Cosmereg
January 21, 2023
Key Developments in EU and UK Regulations: Early 2023 Updates 1. Recent Developments in Chemical Regulations In recent years, there has been growing scrutiny on ...
FDA: 2023 Medical Device User Fees Announcement
Cosmereg
November 2, 2022
FDA: 2023 Medical Device User Fees Announcement Medical device user fees for the Fiscal Year 2023 are now available following an announcement from the U.S. ...
What’s the Difference Between Food and Dietary Supplement Labeling in the United States and Canada?
Cosmereg
July 14, 2022
What’s the Difference Between Food and Dietary Supplement Labeling in the United States and Canada? What’s the Difference Between Food and Supplement Labeling in ...
California Cosmetics Allergens Labeling Requirements
Cosmereg
July 13, 2022
California Cosmetics Allergens Labeling Requirements Do Businesses Have to List Allergens on Cosmetic Labels? An estimated 10% of people experience allergy-related irritation or hypersensitivity from ...
Dietary Supplement Labeling Guide
Cosmereg
July 13, 2022
Dietary Supplement Labeling Guide: Understanding Claims, Labels and Regulations Dietary supplements are in high demand, and businesses offering or planning to offer any of the ...
Health Canada’s Innovative Labelling Requirements for Natural Health Products
Cosmereg
July 10, 2022
Navigating Change: Key Updates in Natural Health Product Labelling Regulations On July 6th, 2022, Health Canada introduced pioneering labeling requirements under the Natural Health ...
US FDA New OTC Monograph User Fee for FY 2022
Cosmereg
July 8, 2022
US FDA OTC Monograph User Fee for FY 2022 The US Food and Drug Administration (FDA) announced new Over-the-Counter (OTC) Monograph consumer fees for ...