REGULATORY NEWS AND RESOURCES
- All
- Canada Cosmetic Regulations
- Canada Dietary Supplement NHP NPN
- Dubai Cosmetic Regulations
- EU Cosmetic Regulations
- US FDA Dietary Supplements
- US FDA food regulations
- US FDA Medical Devices
- US FDA OTC regulations
- USA Cosmetic Regulations
- All
- Canada Cosmetic Regulations
- Canada Dietary Supplement NHP NPN
- Dubai Cosmetic Regulations
- EU Cosmetic Regulations
- US FDA Dietary Supplements
- US FDA food regulations
- US FDA Medical Devices
- US FDA OTC regulations
- USA Cosmetic Regulations
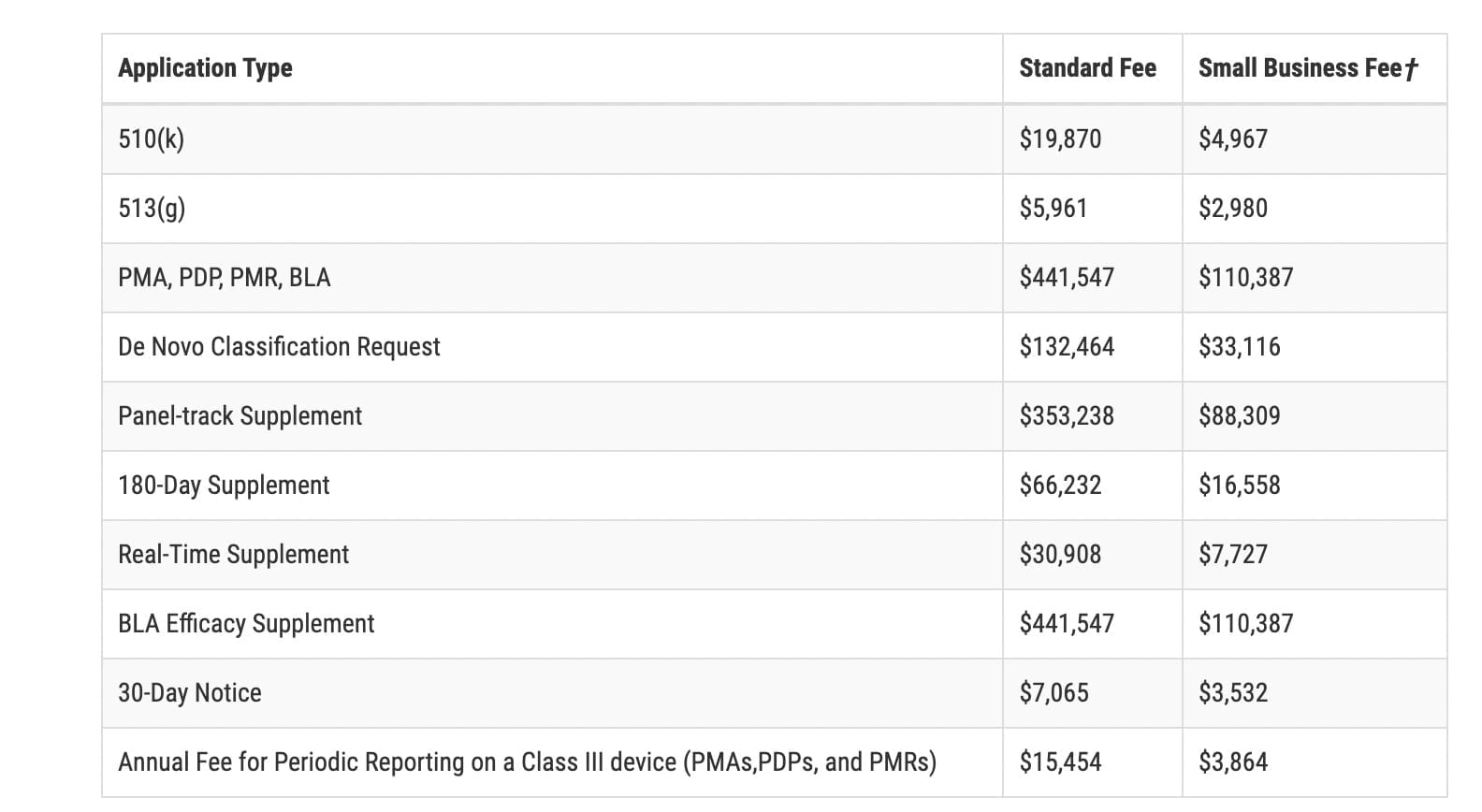
FDA: 2023 Medical Device User Fees Announcement
FDA: 2023 Medical Device User Fees Announcement Medical device user fees for the Fiscal Year 2023 are now available following an announcement from the U.S. …
What’s the Difference Between Food and Dietary Supplement Labeling in the United States and Canada?
What’s the Difference Between Food and Dietary Supplement Labeling in the United States and Canada? What’s the Difference Between Food and Supplement Labeling in …
California Cosmetics Allergens Labeling Requirements
California Cosmetics Allergens Labeling Requirements Do Businesses Have to List Allergens on Cosmetic Labels? An estimated 10% of people experience allergy-related irritation or hypersensitivity from …
Dietary Supplement Labeling Guide
Dietary Supplement Labeling Guide: Understanding Claims, Labels and Regulations Dietary supplements are in high demand, and businesses offering or planning to offer any of …
Health Canada’s Innovative Labelling Requirements for Natural Health Products
Navigating Change: Key Updates in Natural Health Product Labelling Regulations On July 6th, 2022, Health Canada introduced pioneering labeling requirements under the Natural Health …
US FDA New OTC Monograph User Fee for FY 2022
US FDA OTC Monograph User Fee for FY 2022 The US Food and Drug Administration (FDA) announced new Over-the-Counter (OTC) Monograph consumer fees for …
3 Food Labeling Violations You Can Easily Avoid
3 Food Labeling Violations You Can Easily Avoid The U.S. Food and Drug Administration (FDA) has strict controls in place over food companies. …
Over the counter drug registration in the US
Over the counter drug registration in the US An OTC drug or over-the-counter drug is a product that has generally been considered safe to use …
EU Cosmetic Ingredients Ban Extends to Zinc Pyrithione
EU Cosmetic Ingredients Ban Extends to Zinc Pyrithione Zinc Pyrithione is a common anti-dandruff ingredient that has been added to the EU cosmetic ingredients …